Which of the Following Statements Correctly Describe Specific Heat Capacity
Since the system was isolated there was no energy exchanged with the environment. Thus the correct option is D.
Specific Heat Capacity Video Lessons Examples Step By Step Solutions
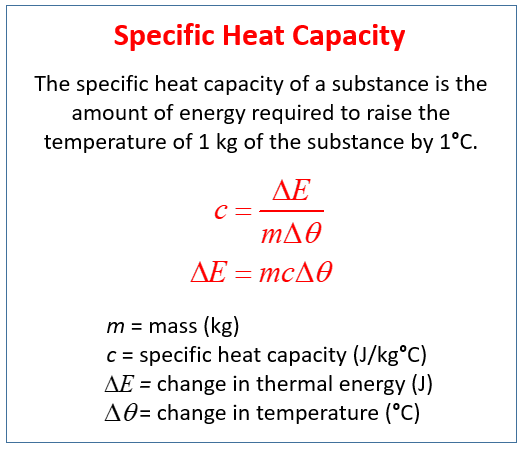
Q m x C x triangleT.
. Select all that apply. Dieter Haemmerich in Principles and Technologies for Electromagnetic Energy Based Therapies 2022. For example the lower specific heat capacity of fat compared to other soft tissue indicates that fat requires.
Q nCP ΔT q n C P Δ T. Match each term in this equation with its meaning. Heat capacity is directly proportional to the mass of an object.
1 The specific heat capacity of iron is approximately half that of aluminum. Select all statements that correctly describe the information conveyed by the following balance chemical equation. Two balls of equal mass one made of iron and the other of aluminum both at 90 C are dropped into a thermally insulated jar that contains an equal mass of water at 25 C.
You are certain that the metal is either copper specific heat 0093 calg C lead specific heat 0031 calg C or aluminum specific heat 022 calg C. Which of the following statements correctly describe exothermic and endothermic reactions. If the temperature of a certain mass of aluminum having a specific heat capacity of 08 J g o C is lowered by 8 o C and the heat lost is 256 J then the mass of the aluminum is.
The symbol for specific heat is c p with the p subscript referring to the fact that specific heats are measured at constant pressure. Which of the following statements does NOT correctly describe buffer capacity. The statement that best defines specific heat would be the one that specifies it as the amount of heat required to increase the temperature of 1 g of a substance by 1 C.
Specific heat capacity defines the relationship between heat and density for a given substance. Specific heat gives the heat capacity per kilogram of a substance. Specific heat capacity defines the relationship between heat and density for a given substance.
Heat Q2 transferred into the fluid m2. The specific heat is the heat capacity for one mole of substance. The SI unit used to measure specific heat capacity is expressed as calories per gram degrees Celsius calg C - I chose answer B.
Which of the following statements best describes specific heat. Specific Heat Capacities Material Jg C calg C Aluminum 0900 0215 Copper 0386 0092 Lead 0128 0031 40. Solution to a Because the water is in thermal contact with the aluminum the pan and the water are at the same temperature.
Specific heat values for solids will never vary for different ranges of temperature. Which of the following is accurate when discussing specific heat. Seeing Specific Heat and Latent Heat Specific heat capacity is the measure of the heat energy required to raise the temperature of a given quantity of a substance by one kelvin.
Hotter at the bottom c. Given the following thermochemical data at 1 atm and knowing that the standard enthalpies of. Q1 Q2 0.
Q 20 mol752 J molK10 K q 20 mol 752 J m o l K 10 K q 1504J q 1504 J. Heat Q1 transferred out of the fluid m1. Select the statement that correctly defines the actual yield of a chemical reaction.
Calculate the temperature difference. Specific heat capacity is the same per unit mass for any substance. The mass m specific heat c change in temperature ΔT and heat added or subtracted Q are related by the equation.
First shell 2 second shell 8 third shell 7 Which of the following is a diatomic molecule. The Qs can be represented by m DT. Table below lists the specific heats of some common substances.
The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by 1C. The specific heat of a gas may be measured at constant pressure. Definition of Specific Heat and Heat Capacity.
Heat capacity gives the amount of energy required to raise the temperature of a given sample of a substance by 1 o C. For simplicity assume that there is no heat loss to the air. Δ T T f T i.
Select all that apply. Which of these statements correctly describe the basic concepts and uses of VSEPR theory. C m S.
Which of the following statements does NOT correctly describe what happens when a hot block is placed in contact with a cool block. The specific heat capacity c Jkg K of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K 1C. Values of specific heat are dependent on the properties and phase of a.
By definition the specific heat capacity of a substance is the quantity of heat required to raise a unit mass in gram of the substance by a unit temperature. Specific heat and heat capacity are related by mass. Where C is heat capacity m is mass of a material and S is specific heat.
Alternatively the equation may be written. The units for specific heat can either be joules per gram. Note that since specific heat is per unit mass its value does not change no matter the size of the sample.
131 Specific heat capacity. If 83680 J of heat are required to raise the temperature of 4 kg of water by 5 o C then the specific heat capacity of water in J kg o C is. Use the equation for specific heat capacity to determine the function needed to solve for the variables associated with temperature change heat c mass deltaT mass q c deltaT.
Cooler at the bottom d. It depends on the rate of flow of water. The specific heat values for water and aluminum are given in the previous table.
Consider the following well-known equation. The heat capacity and the specific heat are related by Ccm or cCm. It is an intensive property.
The temperature T3 was greater than T2 so the mass of fluid m2 had an increase in its internal energy. Which of the following statements correctly describe specific heat capacity c. Plants have nervous system that.
Specific heat does not depend on the mass of an object. The units of specific heat are JgK. Specific heat values for liquids will never vary for different ranges of temperature.
The specific heat of a gas can be measured at constant volume only.
Lesson Specific Heat Capacity Nagwa

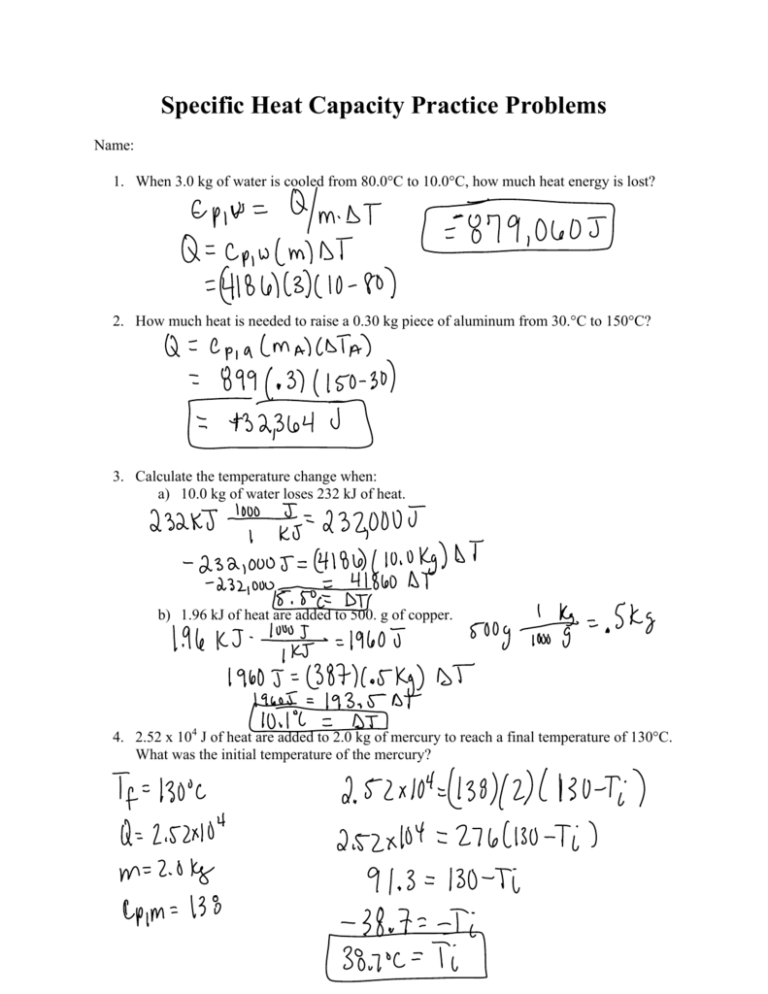
Specific Heat Capacity Practice Problems
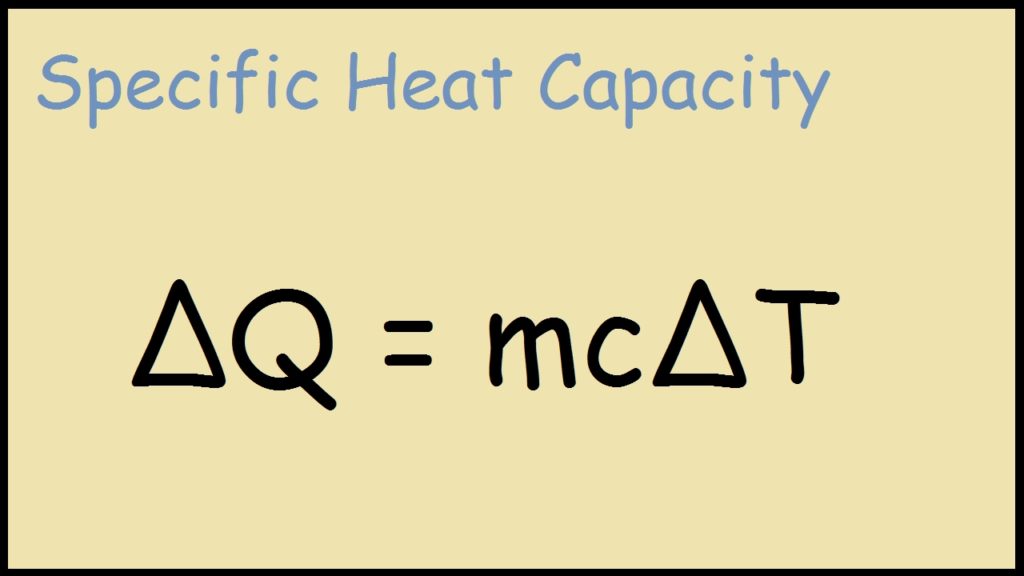
Specific Heat Formula Definition Equations Examples
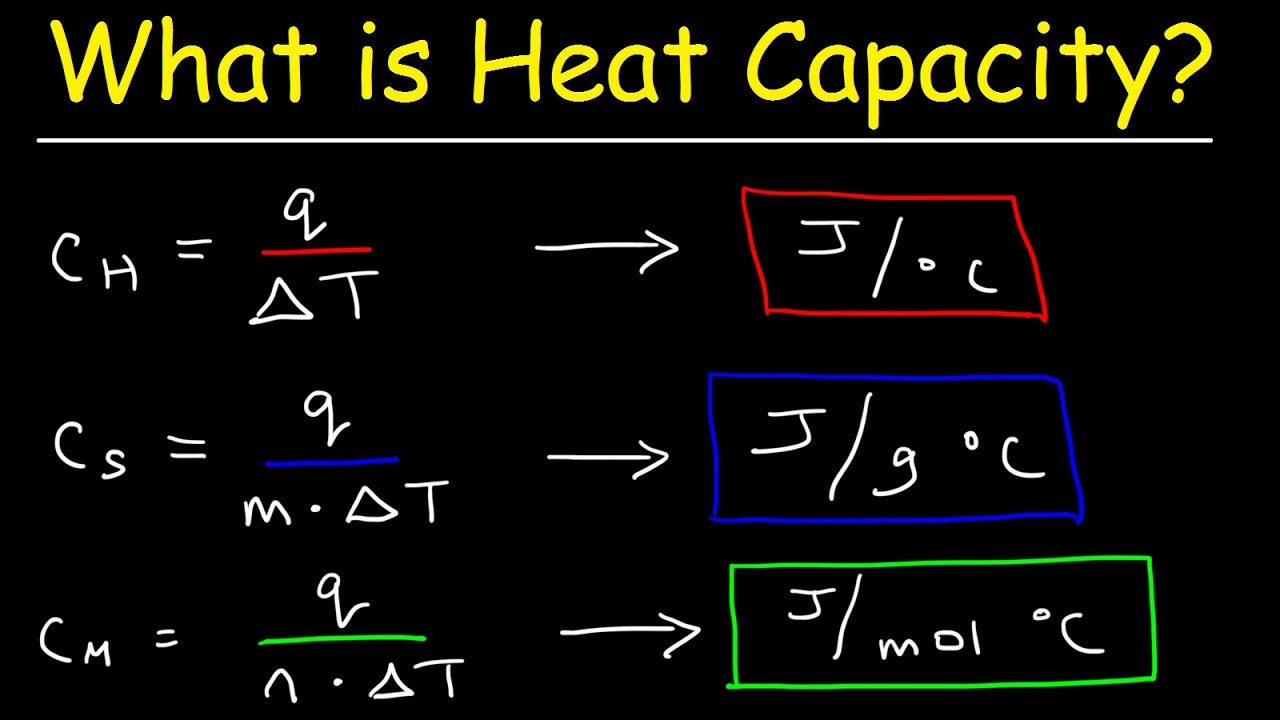
What Is The Difference Between Specific Heat Capacity Heat Capacity And Molar Heat Capacity Youtube
No comments for "Which of the Following Statements Correctly Describe Specific Heat Capacity"
Post a Comment